Catalyst
Catalysts play an important role in the production of chemicals and compounds, significantly affecting, enhancing, or accelerating the reactions between two or more substances to produce a distinct end product. Activated Carbon, widely employed either as a catalyst carrier or as a direct catalyst in numerous applications, is a prime example. Its fundamental purpose is to facilitate oxidation/reduction (REDOX) reactions by taking advantage of its extensive conjugated system that efficiently stabilizes free radicals. This catalytic activity is essential for optimizing manufacturing rates and volumes across various industries, including the alteration of compounds to lessen their impact on processes, environmental health, and human well-being. Enhancements in the catalytic activity of Activated Carbon are achieved through modifications to its surface chemistry, increasing its effectiveness in promoting REDOX reactions.
The industry makes general use of two key types of catalysts today: support catalysts and true catalysts. Catalysts themselves can be classified into two main categories based on their phase relative to the reactants: either Homogeneous or Heterogeneous.Some of the key industrial applications include:
Glyphosate Herbicide
Specially designed activated carbon is a catalyst in manufacturing glyphosate, a biodegradable herbicide.

Precious Metal Catalyst Carrier
precious metals in pharmaceutical and chemical synthesis, optimizing catalyst
use and reducing production time.

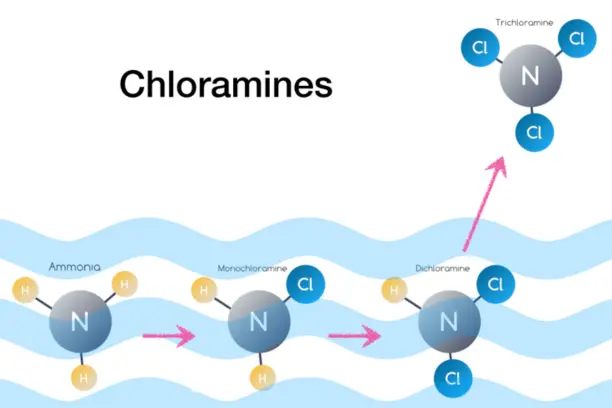
Chloramines
sulfide in potable, process and other waters and peroxide destruction.
Additionally, it excels in removing low molecular weight organic compounds and
the by-products of water chlorination, such as chloroform and trihalomethanes (THM).